Common Clinical Findings Uncovered Through Urinary Metabolite Testing
By: Stacy Hinz, MS

There are many reasons to use 24-hour comprehensive urinary testing in assessing hormones and metabolites in the steroidogenic pathways, but most practitioners choose comprehensive urinary hormone testing for the following reasons:
- Urinary hormones are a direct measurement of the active free hormone available to the tissue receptors. In serum, the amount of hormone available to the tissue receptor is estimated based on binding calculations and population assumptions.1,2
- By testing over a larger time period and normalizing daily hormone fluctuations, urinary hormone testing prevents false highs and lows that can show up when testing in more immediate, moment-in-time methods of detection like serum and saliva.
- Urinary hormone testing provides a more in-depth clinical picture due to the ability to measure hormone metabolites along the steroidogenic pathway. These metabolites indicate how the primary hormones are affected by enzymes and cofactors between hormone synthesis and hormone elimination.
This deeper dive into the clinical utility of urinary hormones and metabolites will be the focus of this article. As the primary hormones and hormone metabolites move along the steroidogenic pathway, urinary testing can trace imbalances that are contributing to common clinical issues by assessing key indicators along the pathway. The result is a clearer clinical picture and greater ability to monitor subsequent therapies and lifestyle changes.
This article will describe some of the benefits of urinary hormone testing and will highlight specific cases where common clinical findings, (often difficult to identify in serum or salivary testing) were easily uncovered, and even predicted, by measuring primary hormones and their metabolites in aging adults.
Progesterone Metabolites
Why one dose does not fit all
It is a common clinical question when prescribing progesterone to different patients with similar progesterone levels; why do some still suffer from anxiety and insomnia, while some are too sedated or tired, and some respond as expected? The answer lies in the way the patient metabolizes progesterone. Progesterone metabolizes to 5a-pregnanediol and 5b-pregnanediol at varying levels. The amount of each is dependent upon the amount of 5-alpha or 5-beta-reductase activity.3–5 By examining the metabolites of progesterone and assessing other metabolism pathways of 5-alpha-reductase activity, practitioners can predict how a patient may respond to progesterone therapy and can also modify the pathways of progesterone metabolism for the best clinical response.
Total Estrogen Analysis vs Estradiol Alone
Uncovering estrogen dominance in patients with low estradiol
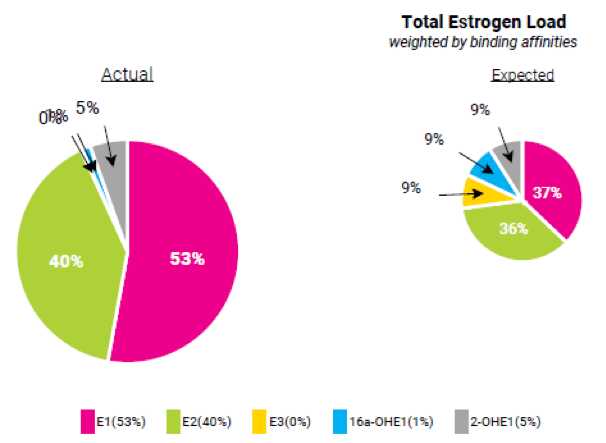
Aromatase activity is responsible for converting androstenedione to estrone as well as testosterone to estradiol. In younger adults, estradiol is the primary estrogen produced. However, as patients age, estrone becomes the primary estrogen produced.1 This transition from more estradiol to more estrone presents an issue when measuring and monitoring estrogen in aging patients because many practitioners only use estradiol to monitor estrogen levels and aging patients can falsely appear estrogen deficient when estradiol is the only estrogen measured. Measuring only estradiol is not advisable when deciding if premenopausal woman, or even postmenopausal woman, need estrogen therapy because many aging women with estrogen dominance or elevated estrogens can still have low estradiol levels. Placing this category of women on estrogen therapy could potentially lead to worsening symptoms and even endometrial cancer risk.2,6,7 Additionally, metabolites of estrone and estradiol can indicate certain risk factors and often have estrogenic strength as well, so it is important to know the total effect E1, E2, E3 and their metabolites are having on the target tissues.
Physicians Lab provides a Total Estrogen Load in each of its urinary hormone testing profiles (Figure 1), so it is easy to assess total estrogen effect in the patient. Each estrogen is weighted by its expected binding affinity at the estrogen receptor and is also presented in a helpful pie chart that compares expected relative levels with the levels observed in the patient. In men, estrogen is assessed vs testosterone, and in women, estrogen is assessed vs progesterone to identify estrogen dominance and uncover any imbalanced between these metabolic pathways.
Aromatase Activity
Pathways to estradiol and estrone
Aromatase activity converts testosterone to estradiol or androstenedione to estrone. While increased aromatase activity can be treated with an aromatase inhibitor, aromatase inhibitors can dramatically decrease estrogen and result in heart issues as well as declining brain and bone health.3,7,8 Monitoring and understanding the pathways of aromatase can assist practitioners in identifying areas of improvement with or without aromatase inhibitors. Aromatase activity is easily monitored in urine.
Aromatase to Estradiol
In this male patient (Figure 2), we can see increased aromatase activity indicated in several areas.
- Estradiol is dominating the total estrogen load
and is higher than expected, in relative ratio to the other estrogen components
of the total.
- This means that aromatase activity is predominantly aromatizing testosterone to estradiol
- His elevated testosterone/estrogen ratio with decreased metabolism to downstream metabolites shows that testosterone is metabolizing toward estradiol more often and with more preference than downstream metabolites of testosterone
Testosterone must be metabolized and eliminated. There are essentially two pathways for this: the 5a-reductase pathway (creating testosterone metabolites) or the aromatase pathway (creating estradiol). When testosterone is elevated, often aromatase activity will often increase, and the level of expected testosterone metabolites will decrease (which is the case with this patient). When this happens, the ratio between testosterone and estrogen will indicate estrogen dominance. This means that aromatase activity is creating an imbalance between testosterone and estrogen. Aromatase activity and 5a-reductase activity are often negatively correlated, because when 5a-reductase goes up, there is less testosterone available for aromatase activity, and vice versa. However, this is not always the case. Sometimes when 5a-reductase activity is low, testosterone will start to pool and appear elevated due to the inability to metabolize downstream and the excess testosterone will not aromatize. Therefore, we examine the ratios of testosterone to estrogen and testosterone to its downstream metabolites in order to determine which endpoint of testosterone is dominating.
- Symptoms of estrogen dominance
- Although the testosterone is elevated, the patient is struggling with symptoms that mimic testosterone deficiency because estrogen is having a greater effect at the tissues. This is common in patients with estrogen dominance.
Not every male with elevated aromatase activity will have high levels of testosterone with elevated estrogen (like the patient described here), so it is important to have multiple measures with steroid pathway metabolites when interpreting and assessing aromatase activity pathways.
By examining the pathways and measuring multiple points along these pathways of hormone synthesis and metabolism, it becomes clear where certain pathways may have accelerated and others may have decreased.

Aromatase to Estrone
In a similar example, aromatase activity to estrone is the conversion of androstenedione to estrone (instead of testosterone to estradiol), so there is a different mechanism to examine when aromatase activity results in increased estrone. Androstenedione originates from DHEA and progesterone pathways of metabolism. As mentioned previously, estrone is the dominant estrogen in aging men and women; but what mechanisms cause estrone to increase and estradiol to decrease in aging patients?

Testosterone to estradiol conversion happens at the sex organs in younger adults whereas androstenedione conversion to estrone happens in adipose tissue and adrenals as well as the sex organs.9 As patients age, the ovaries and testicles decline in function causing lower testosterone production, increased androstenedione production and increased conversion of androstenedione to estrone vs. testosterone.8 Androstenedione is the predominate steroid hormone produced by the postmenopausal ovary in women and aging patients often have inflammatory responses and other issues causing the adrenals to produce more androstenedione as well. The presence of more androstenedione is not the only reason for increasing levels of estrone – the declining expression of certain enzymes required for testosterone production in the steroidogenic pathway pushes metabolism of androstenedione directly to estrone instead of testosterone.10
Androstenedione is primarily a product of DHEA but can also be created from progesterone, so when increased aromatase activity results in estrone, it is important to check both DHEA and progesterone levels (especially in patients taking DHEA or progesterone supplementation).
When deciding how to treat patients with increased aromatase activity, there are several options to consider. Assessing the multiple markers along the pathway, as well as examining which pathway of metabolism seems to be predominant, is crucial. Increasing testosterone, decreasing testosterone, increasing DHEA, decreasing DHEA, decreasing progesterone, or inhibiting aromatase activity are all options. However, choosing the best options requires an understanding of the pathway of metabolism.
Hypothyroidism and Estrogens
Linking aromatase activity, thyroid activity and cortisol metabolism
The conversion of androstenedione to estrone opens the topic of how hypothyroidism affects estrogen levels. Hypothyroid decreases the rate of metabolism to androstenedione, thereby decreasing the amount of estrone produced.11,12 This can cause irregular periods in premenopausal women and can dramatically reduce the total estrogen load in postmenopausal women who primarily produce estrone from androstenedione so hypothyroidism can decrease estrone production. Did you know that hypothyroidism is often caused by estrogen excess/dominance? The human body relies so much upon maintaining balance that excess estrogen can cause hypothyroidism, which then has the ability to reduce estrogen production. This is one of many mechanisms that reminds us how crucial balance is, how the body tries to correct itself and why it is so important to know which pathways of metabolism are being affected before attempting to achieve and maintain balance during therapy.
When examining urinary hormones in the HPA-Axis, low metabolism of cortisol is a good first indicator of hypothyroidism.11 As you can see in the same male patient from Figure 2, estrogen dominance is combined with low metabolism of cortisol (Figure 3). While estrogen dominance is not the only thing that can cause hypothyroidism, we can certainly see that this patient’s elevated testosterone, low testosterone/estrogen ratio, and decreased 5a-reductase activity are clear indicators of increased aromatase activity. This pattern is almost always associated with the reduction in cortisol metabolism, which indicates hypothyroidism, and shows why elevated aromatase activity is almost always associated with insulin resistance.13 Simply reducing testosterone and prescribing an aromatase inhibitor may benefit the patient as long as all of the balancing measures are closely monitored during treatment.

Male Testosterone Pellet Therapy Issues
Avoiding 2nd and 3rd pellet disappointment in men
How many times do male patients feel a significant improvement in energy, libido and overall wellbeing after the first testosterone pellet, only to be faced with declining benefits in the 2nd and 3rd pellet insertion? Monitoring testosterone in serum can show misleading moderate testosterone results and tracking estrogen levels in serum can mask increased aromatase activity. The male patient described had this experience due to elevated levels of testosterone and elevated levels of aromatase activity. The patient was experiencing symptoms of low androgens, even though the testosterone levels were adequate in serum. The patient insisted that he was ready for a higher dose of testosterone, and when the practitioner measured serum the testosterone levels justified a slight increase. Unfortunately for this patient, the free testosterone levels in urine would have indicated elevated testosterone, but the calculated serum testosterone levels (not a direct measurement of free) indicated room for more therapy. This patient already had some symptoms of testosterone excess such as acne, hair loss, irritability and aggression, but the patient was more concerned about the reoccurrence of other symptoms like erectile dysfunction, depression, anxiety, fatigue, and weight gain which he associated with low testosterone. In fact, these additional symptoms were associated with excess estrogen caused by increased aromatase activity. What appeared to be declining effects of testosterone, turned out to be increasing effects of estrogen with symptoms of testosterone excess. Additionally, the increased aromatase activity triggered lower cortisol metabolism (associated with hypothyroidism) accompanied by hypothyroid symptoms, which can also mimic low testosterone. Although the serum testing and the patient’s symptoms appeared to warrant higher levels of testosterone therapy, urinary hormone testing showed that this patient needed a different approach to reach and maintain balance between testosterone and estrogen for the best clinical outcome.
Therapy:
- Using an aromatase inhibitor to reduce the amount of estrogen produced and help with energy levels and sexual function.
- Supporting Phase I and Phase II detoxification of estrogens to help eliminate excess estrogen down the most favorable pathways of metabolism and mitigate the risks associated with elevated levels of estrogen in men.
- Reducing testosterone therapy addressing acne, hair loss, and mood.
- The reduction in aromatase activity and detoxification of excess estrogen should improve the sluggish cortisol metabolism and help reverse the signs of hypothyroidism. However, a healthy-thyroid lifestyle and/or thyroid support will also help the transition back to balance
Metabolic Syndrome, Type II Diabetes and the HPA-Axis
Addressing imbalances in metabolic pathways
As already mentioned, low metabolism of cortisol is a good first indicator of hypothyroidism. This makes sense when you consider the connection between cortisol, insulin resistance and thyroid. However, when we see elevated cortisol metabolism, we experience insulin resistance issues as well.13,14 For example; this patient appears to have low cortisol levels throughout the day with an unexpected increase in the bedtime sample (Figure 5). We confirm, through cortisone results, that the cortisone has a similar trend but at higher levels than cortisol, which is a first clue of increased metabolism. When we look at the cortisol metabolites, they appear elevated and the cortisol:cortisol metabolite ratio appears low. That indicates high cortisol metabolism, so we can see that even though the cortisol levels appear predominantly low, the adrenal glands are pumping out cortisol and this patient is metabolizing that cortisol at elevated rates. This pattern is very typical of someone who is at a high risk of Type II diabetes.13 In fact, new studies indicate that the pattern of elevated cortisol metabolism combined with an evening increase in diurnal cortisol was used to predict the onset of Type II diabetes over the course of 9 years with over 70% accuracy (refs supporting these numbers).
We know that abdominal obesity is linked to both Type II diabetes and elevated cortisol metabolism. We also know that Type II diabetes is one of the qualifiers for metabolic syndrome, so it makes sense that a the warning signs of type II diabetes, insulin resistance and metabolic syndrome can all be identified by examining the cortisol metabolites and the anabolic:catabolic ratio. It should also make sense that if we can identify these warning signs in urine, there are likely other metabolic issues that can be addressed by measuring, achieving and maintaining the delicate balance between the anabolic and catabolic metabolism pathways.

The Anabolic vs Catabolic Ratio
Measuring, achieving and maintaining metabolic balance in the HPA-axis
The anabolic to catabolic ratio compares HPA-Axis balance between 17-ketosteroids (mostly DHEA metabolites) and 17-hydroxysteroids (mostly cortisol metabolites). The easiest way to understand the anabolic/catabolic ratio is to view it as a balance between creating energy (through catabolic processes) and using that energy to build (through anabolic processes).
In order to build and maintain tissues, the body requires energy. This energy is created by breaking down (catabolizing) energy storage sources such as carbohydrates, fats and proteins.15 If the catabolic pathways are balanced with the anabolic pathways, then we end up with the perfect amount of energy generated by the catabolic pathway and utilized by the anabolic pathway.16 When the catabolic pathway is higher than the anabolic pathway, we end up with more stored energy – often in the form of fat – and run the risk of Type II diabetes and metabolic syndrome.17 Although we focus a large deal on low anabolic/catabolic ratios where cortisol metabolism and 17-hydroxysteroids are dominating over anabolic metabolism and 17-ketosteroids, there are plenty of cases where the anabolic pathways dominate the catabolic pathways as well. While this can be less common, it is in no way less harmful to the patient. For these patients, they have the ability to build but they lack the energy to do so. When the anabolic pathway is higher than the catabolic pathway, patients are often hypoglycemic, underweight, fatigued and require more caffeine. Due to craving carbohydrates, a percentage of patients may be overweight with muscle loss because their energy sources are more carbohydrate and muscle than fat. These are generalizations for understanding the anabolic to catabolic ratio, but it points out how balance is best.
Elevated Cortisol Metabolism
When cortisol metabolism is increased, this will often result in elevated 17-hydroysteroids and an imbalance in the anabolic to catabolic ratio leaning toward catabolic. When 17-hydroxysteroids increase, balancing the ratio should include slowing the rate of adrenal output – even when diurnal cortisols appear normal or low, because elevated 17-hydroxysteroids directly correlate to increased adrenal output. Temporarily increasing DHEA is also a valid consideration and allows for balance to be achieved between anabolic and catabolic while the patient works on lifestyle improvements to lower cortisol production. Increasing DHEA, even just temporarily, often works well because an imbalance (leaning toward catabolic) can make lifestyle changes, such as weight loss, nearly impossible due to the fact that elevated cortisol metabolites are driving energy storage and weight gain. DHEA therapy can also increase 5-alpha-reductase activity which is often helpful in reducing cortisol metabolism because decreased 5a-reductase is often associated with increased cortisol metabolism as well. Although increasing DHEA is not always an option, DHEA replacement is usually a good option when an imbalance exists due to low 17-ketosteroids and decreased 5a-reductase activity.
Lower 17-ketosteroids
Understanding where the decreased 17-ketosteroids are stemming from in the pathway is important in choosing therapeutic options. To accomplish this, the 17-ketosteroids are displayed in a pie chart in the report allowing for the comparison between the patient results (actual) and the expected results in each individual ketosteroid. Through this graphic, accelerated or sluggish pathway mechanisms can be quickly identified. For example, the patient in Figure 6 has an anabolic to catabolic ratio that is leaning toward the catabolic side. The results indicate both elevated catabolic metabolites and low anabolic metabolites combining to produce an extremely imbalanced ratio. The root cause of the extremely low 17-ketosteroids is due to low DHEA. The cause of the increased cortisol metabolites is not obesity (the patient is only 120 lbs.), but rather metabolism down the 5b-reductase pathway at a much higher rate than expected, which is also associated with increased cortisol metabolism.

In these cases, we can often increase DHEA, which also increases 5a-reductase activity,7,17 and consider slowing 5β-reductase activity using licorice.18 One other benefit of licorice is that it will slow the rate at which back-door androgen metabolism occurs,10 (which is not an issue in this patient) and will assist in sending DHEA metabolism down the 17-ketosteroid pathway. We may also see increases in testosterone and estrogen as DHEA and 5a-reductase activity increase. The end result will be more balance between the anabolic and catabolic in the ratio, both by increasing the anabolic side through DHEA supplementation and decreasing the catabolic side by calming the adrenals and reducing 5b-reductase activity.
CONCLUSION
Physicians Lab’s comprehensive report was designed to take some of the guesswork out of interpreting results and treating patients. The totals and ratios are a good start and the dynamic text contained in these reports can often tell you exactly what is happening because the text is based on the results of each patient.
Our primary focus is to deliver the most accurate results with the highest amount of clinical information and our comprehensive report containing results-driven text allows us to transfer this information directly to the report as the scientific community gains more insight into these pathways.
REFERENCES
1. Roos J, Johnson S, Weddell S, et al. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept Reprod Heal Care. 2015;20(6):438-450. doi:10.3109/13625187.2015.1048331
2. Maskarinec G, Beckford F, Morimoto Y, Franke AA, Stanczyk FZ. Association of estrogen measurements in serum and urine of premenopausal women. Biomark Med. 2015;9(5):417-424. doi:10.2217/bmm.15.10
3. de Ronde W, de Jong FH. Aromatase inhibitors in men: Effects and therapeutic options. Reprod Biol Endocrinol. 2011;9(1):93. doi:10.1186/1477-7827-9-93
4. Starka L, Hampl R, Bicikova M, Jelinek R, Doskovil M. Observations on the biological activity of epitestosterone. Physiol Res. 1991;40(3):317-326.
5. Hiipakka RA, Zhang HZ, Dai W, Dai Q, Liao S. Structure-activity relationships for inhibition of human 5α-reductases by polyphenols. Biochem Pharmacol. 2002;63(6):1165-1176. doi:10.1016/S0006-2952(02)00848-1
6. Lu LJW, Cree M, Josyula S, Nagamani M, Grady JJ, Anderson KE. Increased urinary excretion of 2-hydroxyestrone but not 16α- hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res. 2000;60(5):1299-1305.
7. McCann SE, Edge SB, Hicks DG, et al. A pilot study comparing the effect of flaxseed, aromatase inhibitor, and the combination on breast tumor biomarkers. Nutr Cancer. 2014;66(4):566-575. doi:10.1080/01635581.2014.894097
8. Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004;89(3):1174-1180. doi:10.1210/jc.2003-031467
9. Strauss JF. Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome. Ann N Y Acad Sci. 2003;997:42-48. doi:10.1196/annals.1290.005
10. Fiandalo M V., Wilton J, Mohler JL. Roles for the backdoor pathway of androgen metabolism in prostate cancer response to castration and drug treatment. Int J Biol Sci. 2014;10(6):596-601. doi:10.7150/ijbs.8780
11. Hoshiro M, Ohno Y, Masaki H, Iwase H, Aoki N. Comprehensive study of urinary cortisol metabolites in hyperthyroid and hypothyroid patients. Clin Endocrinol (Oxf). 2006;64(1):37-45. doi:10.1111/j.1365-2265.2005.02412.x
12. Taniyama M, Honma K, Ban Y. Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: Application for diagnosis of resistance to thyroid hormone. Thyroid. 1993;3(3):229-233. doi:10.1089/thy.1993.3.229
13. Westerbacka J, Yki-Järvinen H, Vehkavaara S, et al. Body Fat Distribution and Cortisol Metabolism in Healthy Men: Enhanced 5β-Reductase and Lower Cortisol/Cortisone Metabolite Ratios in Men with Fatty Liver. J Clin Endocrinol Metab. 2003;88(10):4924-4931. doi:10.1210/jc.2003-030596
14. Declue TJ, Shah SC, Marchese M, Malone JI. Insulin resistance and hyperinsulinemia induce hyperandrogenism in a young type B insulin-resistant female. J Clin Endocrinol Metab. 1991;72(6):1308-1311. doi:10.1210/jcem-72-6-1308
15. Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM R. 2011;3(6 Suppl 1):S3-S11. doi:10.1016/j.pmrj.2011.05.009
16. Tzanis G, Dimopoulos S, Agapitou V, Nanas S. Exercise intolerance in chronic heart failure: The role of cortisol and the catabolic state. Curr Heart Fail Rep. 2014;11(1):70-79. doi:10.1007/s11897-013-0177-1
17. Ueshiba H, Shimizu Y, Hiroi N, et al. Decreased steroidogenic enzyme 17,20-lyase and increased 17-hydroxylase activities in type 2 diabetes mellitus. Eur J Endocrinol. 2002;146(3):375-380. doi:10.1530/eje.0.1460375
18. Ferrari P, Sansonnens A, Dick B, Frey FJ. In vivo 11β-HSD-2 activity: Variability, salt-sensitivity, and effect of licorice. Hypertension. 2001;38(6):1330-1336. doi:10.1161/hy1101.096112
